Background: Analysis of bone marrow is the gold standard in patients with unexplainable cytopenia for diagnosing (or excluding) myelodysplastic neoplasm (MDS). Besides morphology also molecular genetics and cytogenetics are currently assessed from BM as both are required for diagnosis and risk stratification. Substitution of BM analysis with peripheral blood (PB) analysis would be a substantial progress especially in the elderly to allow for a less invasive strategy.
Aims: Validate, whether PB reliably reflects molecular and cytogenetic aberrations of BM by evaluating somatic mutations and copy number variations (CNVs) in concurrent PB and BM samples.
Methods: We analyzed paired BM and PB samples (time difference of sampling: <15 days) of 200 cytopenic patients. All samples were subjected to targeted panel sequencing analyzing mutations (assessed as positive at variant allelic frequencies/VAFs >1%) in 40 genes associated with myeloid neoplasms (coverage: 1500x). If available, BM chromosome analysis (CA) and BM FISH analysis were compared to NGS CNV analysis from PB using a human CNV backbone hybridization panel (coverage: 50x).
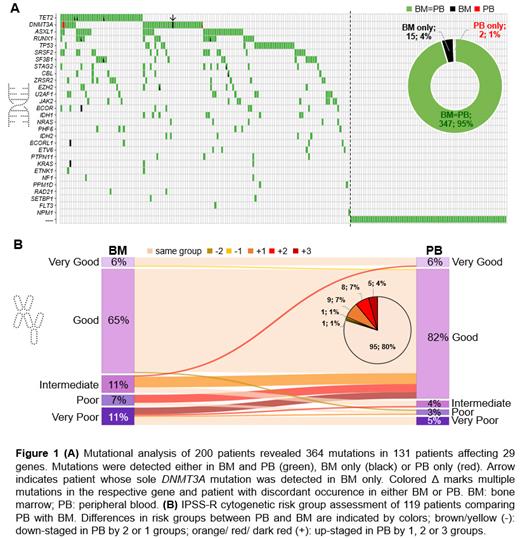
Results: In 69 patients (35%) no mutations (MUTs) were detected in either BM or PB (Figure 1A). For the remaining 131 patients at least one MUT was found in BM (median MUTs/patient: 2; range 1-10). In 130/131 patients MUTs were detected in both BM and PB (same number of MUTs: n=121; higher number of MUTs in BM than in PB: n=9) while in only one patient harboring a DNMT3A MUT as the sole MUT in BM (VAF: 9%) no MUT was detected in PB. This resulted in a negative predictive value of 98.6% (69/70).
Overall, we detected 364 MUTs in 29 of the analyzed genes (BM MUTs: n=362; PB MUTs: n=349) and a concordance between BM and PB of 95% (347/364; Figure 1A). The median VAF was lower in PB compared to BM across all MUTs (median VAF: 20% vs. 12%; p<0.001). All MUTs detected in BM at VAF ≥13% (n=229) were also detected in corresponding PB.
Next, we compared CNVs detected by PB NGS to BM karyotype (KT). For 119/200 patients PB NGS including a CNV-backbone panel was available. BM CA revealed a normal KT in 71 and an aberrant KT in 48 patients. In total, 126 aberrations were detected in BM CA including 7 balanced aberrations that can technically not be detected using the CNV backbone panel. CNV analysis from PB revealed 68 aberrations (of 119 detectable BM CNVs; 54%) with 64 being concordant with BM CA. In total, in 88/119 patients (74%) concordant results were found in BM and PB (70/71 with normal and 18/48 with aberrant BM KT). In 3 patients (2.5%) different CNVs were detected (1/3: no CNV in BM CA), while in 28 (23.5%) either fewer CNVs (n=8; all complex KTs) or no CNVs (n=20) were detected in PB compared to BM. The majority (76%) of BM CNVs not detected by PB NGS with available BM FISH data were small BM clones (clone size ≤30%) and therefore not reliably captured by NGS PB with a technical sensitivity of 30%. Interestingly, 4 of the discordant patients with aberrant BM KT did not harbor any somatic MUT in BM and PB. Regarding the IPSS-R cytogenetic risk group, 95 (80%) patients fell into the same category, while 22 (18%; all with aberrant BM KT) were assigned to a better risk category by NGS PB than based on BM CA data and 2 patients (2%) to a worse risk category by NGS PB (Figure 1B).
Conclusion: In cytopenic patients mutations can be reliably detected from PB showing a concordance with BM of individual mutations of 95% and a consistency regarding overall mutation detection between PB and BM in 99.5% of patients. Cytogenetic risk group assignment based on NGS CNV analysis from PB compared to chromosome analysis from BM resulted in the same risk group in 80% of patients. However, to further decrease the number of discrepant cases, the technical limitations (sensitivity, inability to detect balanced CNVs) of the CNV backbone method might be overcome by increasing the sequencing depth and/or using CD34-enriched PB samples and adding probe sets. Alternatively, FISH on PB smears could be used for CNV detection. Overall, our data indicate a high degree of overlap between PB and BM regarding mutational and cytogenetic analysis in patients with unexplained cytopenia and suggest PB as reliable surrogate especially in patients without any genetic aberrations.
Disclosures
Huber:MLL Munich Leukemia Laboratory: Current Employment. Wossidlo:MLL Munich Leukemia Laboratory: Current Employment. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Meggendorfer:MLL Munich Leukemia Laboratory: Current Employment. Hutter:MLL Munich Leukemia Laboratory: Current Employment. Hoermann:MLL Munich Leukemia Laboratory: Current Employment. Summerer:MLL Munich Leukemia Laboratory: Current Employment. Ruge:MLL Munich Leukemia Laboratory: Current Employment. Baer:MLL Munich Leukemia Laboratory: Current Employment. Kern:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal